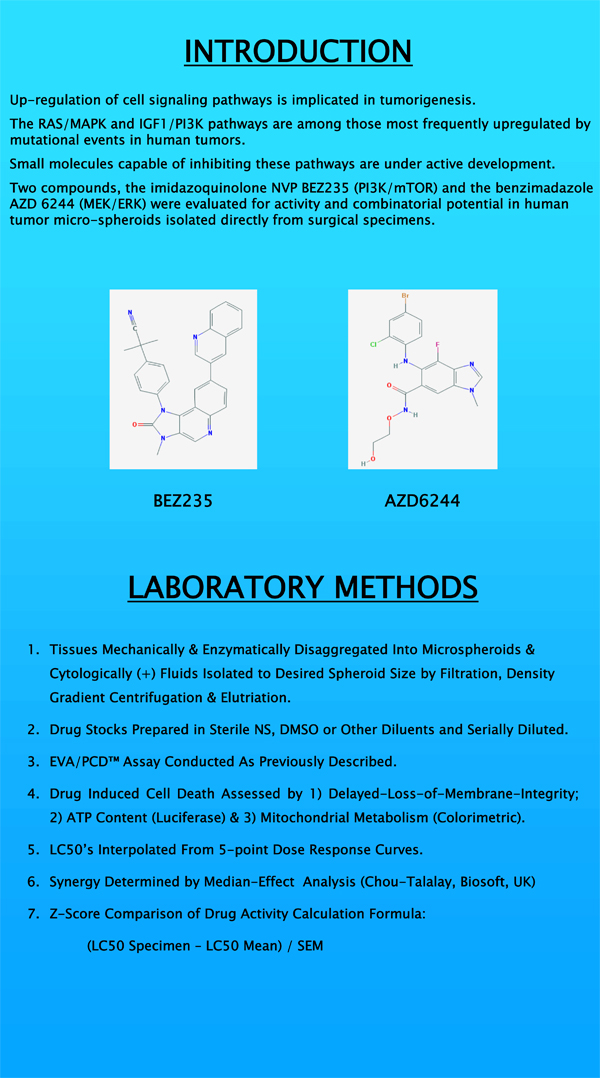
The Sunday, April 3, 2011, experimental and molecular therapeutics session at the AACR 102nd annual meeting included our presentation on signal transduction inhibitors. Using MEK/ERK and PI3K-MTOR inhibitors we explored the activities, synergies and possible clinical utilities of these novel compounds.
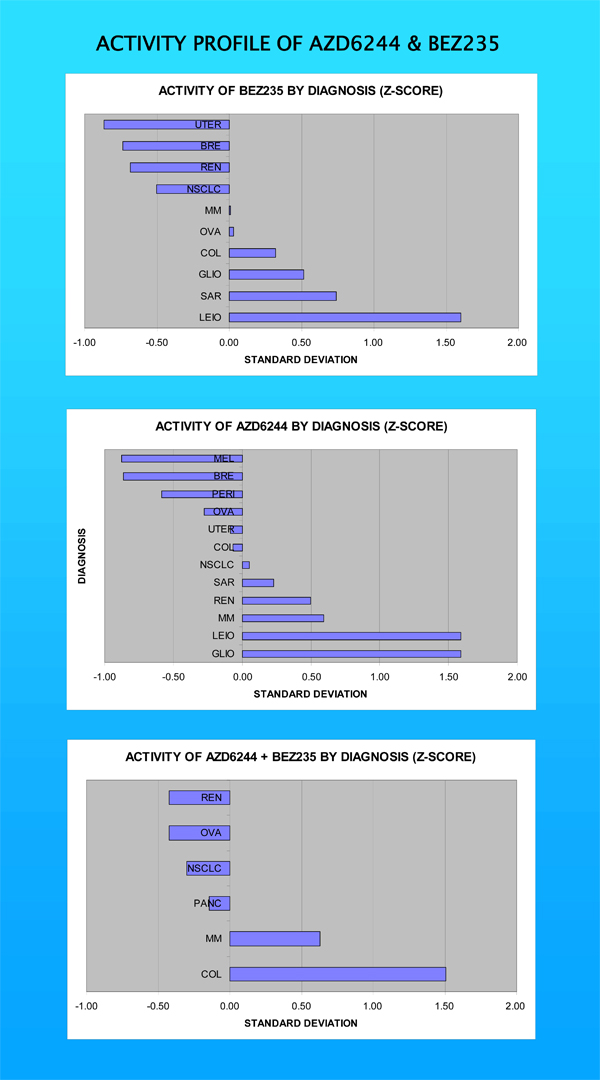
The findings were instructive. First, we saw a good signal for both compounds utilizing the Ex-vivo Analysis of Programmed Cell Death (EVA-PCD) platform. Second, we saw disease-specific activity for both compounds. For the MEK/ERK inhibitor, melanoma appeared to be a favored clinical target. This is highly consistent with our expectation. After all, many melanomas carry mutations in the BRAF gene, and BRAF signals downstream to MEK/ERK. By blocking MEK/ERK, it appeared that we blocked a pathway fundamental to melanoma progression. Indeed, MEK/ERK inhibitors are currently under investigation for melanoma.
For PI3K inhibitors, the highest activity was observed in uterine cancers. This is interest, because uterine carcinomas are often associated with a mutation in the PTEN gene. PTEN is a phosphatase tumor suppressor that functions to block activation of the PI3K pathway. Thus, mutations in the tumor suppressor unleash PI3K signaling, driving tumors to grow and metastasize. Blocking PI3K provided a strong signal, indicating that this approach may be very active in tumors associated with these oncogenic events.
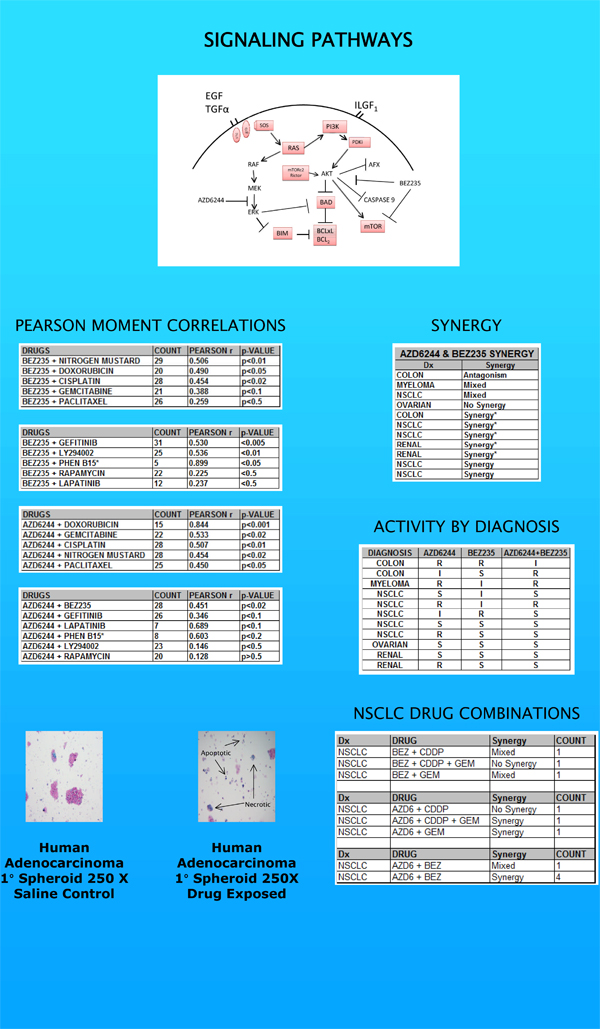
The third point of interest in our report was, perhaps, its most important. Specifically, that we can explore those diseases where MEK-ERK, PI3K and mTOR signaling are less established targets. Cancers of the lung, ovary, colon or breast all manifested profiles of interest. When we combined both pathway inhibitors in a process we call horizontal inhibition, renal cell carcinoma popped up as the best target. These results, though exploratory, suggest a superior approach for drug development, allowing us to identify important leads much faster than the clinical trial process.